-
Call us
+44(0) 1785 878278 -
Mail
info@vitalstim.co.uk -
Timing
09.00am to 5.00pm
Equipment and Supplies for VitalStim® Therapy
NMES devices have been in routine use by rehabilitation professionals for a number of different indications for a long time. A multitude of different stimulators have received 510(k) clearance from the FDA over the years, usually for general indications such as pain relief and muscle strengthening. In 2002 the FDA cleared a modified NMES system comprising a device and electrodes, VitalStim® Therapy, for use for a very specific indication, oropharyngeal dysphagia, and in a very specific anatomical region, the anterior neck.

Unlike in other parts of the body, the use of NMES on the anterior neck has always received a WARNING label from the FDA, indicating that there is risk of serious injury or death when using general clearance NMES devices and supplies on the neck. The reason the FDA issues such strong language is because of unanimous warnings by authors and experts in the field of electrotherapy about the possibility of inadvertently triggering laryngospasm or bradycardia by stimulating the carotid sinus.
Use of NMES devices which have not been cleared for the treatment of dysphagia is contrary to that product’s labeling, is inconsistent with its intended uses, and is beyond the scope of applications implied. Most NMES devices were primarily designed for use in the upper and lower extremities and do not incorporate the software and hardware modifications necessary to ensure safe application of electrotherapy to the anterior portion of the neck.
Implication for the clinician: Use of equipment in high risk anatomical regions such as the anterior neck requires specific clearance from the FDA; general clearance for neuromuscular stimulation/reeducation is not sufficient.
VitalStim® Therapy clearance was obtained after a lengthy process in which FDA first required the manufacturer to submit data to demonstrate safety and efficacy. The FDA also required the manufacturer to submit evidence of design characteristics that would ensure safe and efficacious use of the device and the electrodes by future users (therapists).
Implication for the clinician:Only clinicians who have successfully completed the VitalStim® Therapy Certification Course may administer VitalStim® therapy to patients. Unlike other NMES devices and protocols, VitalStim® is not to be prescribed for independent use in the home by the patient.
Only clinicians who have successfully completed the VitalStim® Therapy Certification Course may administer VitalStim® therapy to patients. Unlike other NMES devices and protocols, VitalStim® is not to be prescribed for independent use in the home by the patient.
FDA Cleared Indication for Use
VitalStim® Therapy System (Device)
"Muscle re-education by application of external stimulation to the muscles necessary for pharyngeal contraction”The VitalStim® Therapy system is the only NMES-based dysphagia treatment to carry this clear and unambiguous indication for use from the FDA.

VitalStim® Therapy Electrodes: Impedance – 23ohms
- Near continuous current delivery versus a 1:1 or even 1:2 duty cycle in typical NMES protocols
- 0.8 inch diameter versus more typical 1 inch or greater
- Long treatment duration of 60 minutes per session versus more typical 20-30 minutes
- Electrodes manufactured in the United States
- Developed specifically for the treatment of dysphagia; not sold for other uses.
Enhance Therapy with sEMG Biofeedback Technology
Interactive Therapy allows patients ti receive visual audible feedback leading to enhanced patient engagement during swallowing exercises. Supplementing a standard therapy program with sEMG Biofeedback facilitates functional swallowing recovery.
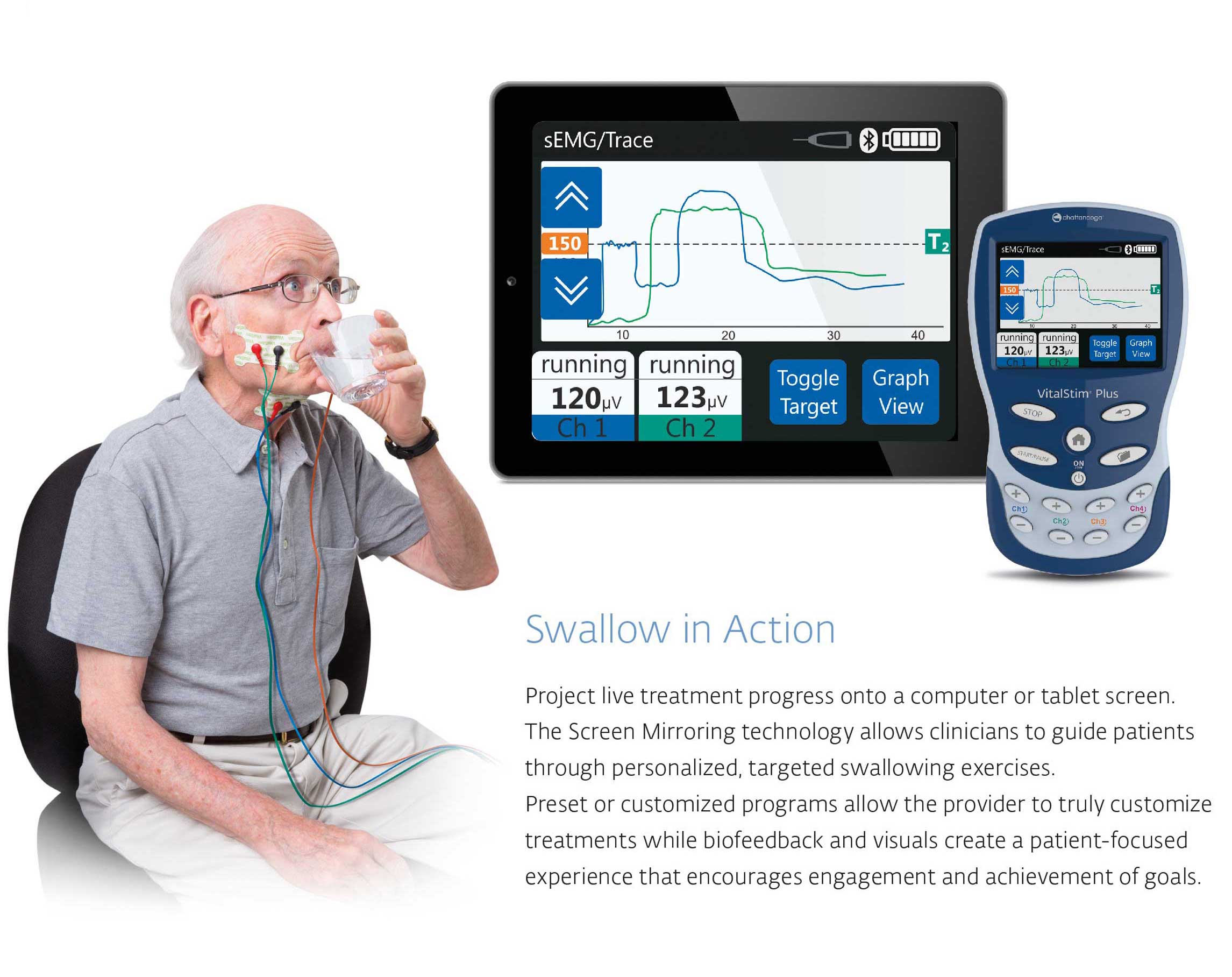
Immediate Patient progress Data
Quantify treatment progression with objective data that demonstrate outcomes for each therapy session
The Taste Of Independence
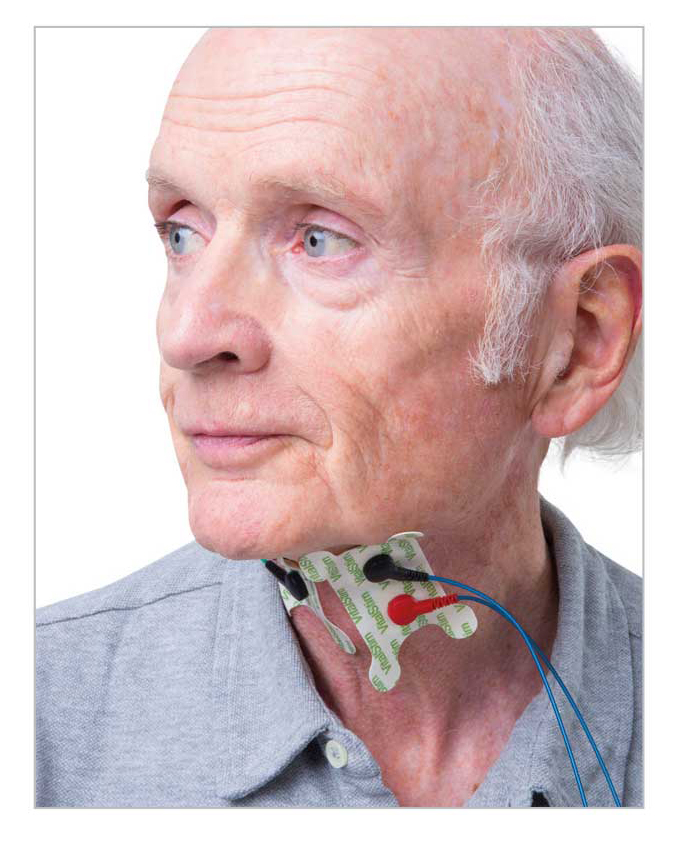
VitalStim plus neuromuscular electrical stimulation helps recruit and re-educate muscles in the process of swallowing. Under the guidance of a clinician,patients partner in an interactive therapy that aids muscle strengthening to rehabilitate Swallowing.
sEMG biofeedback helps to increase effort and duration of swallowing attemptd and to improve coordination .It also offers the potential objective evaluation of swallowing function and patterns.2
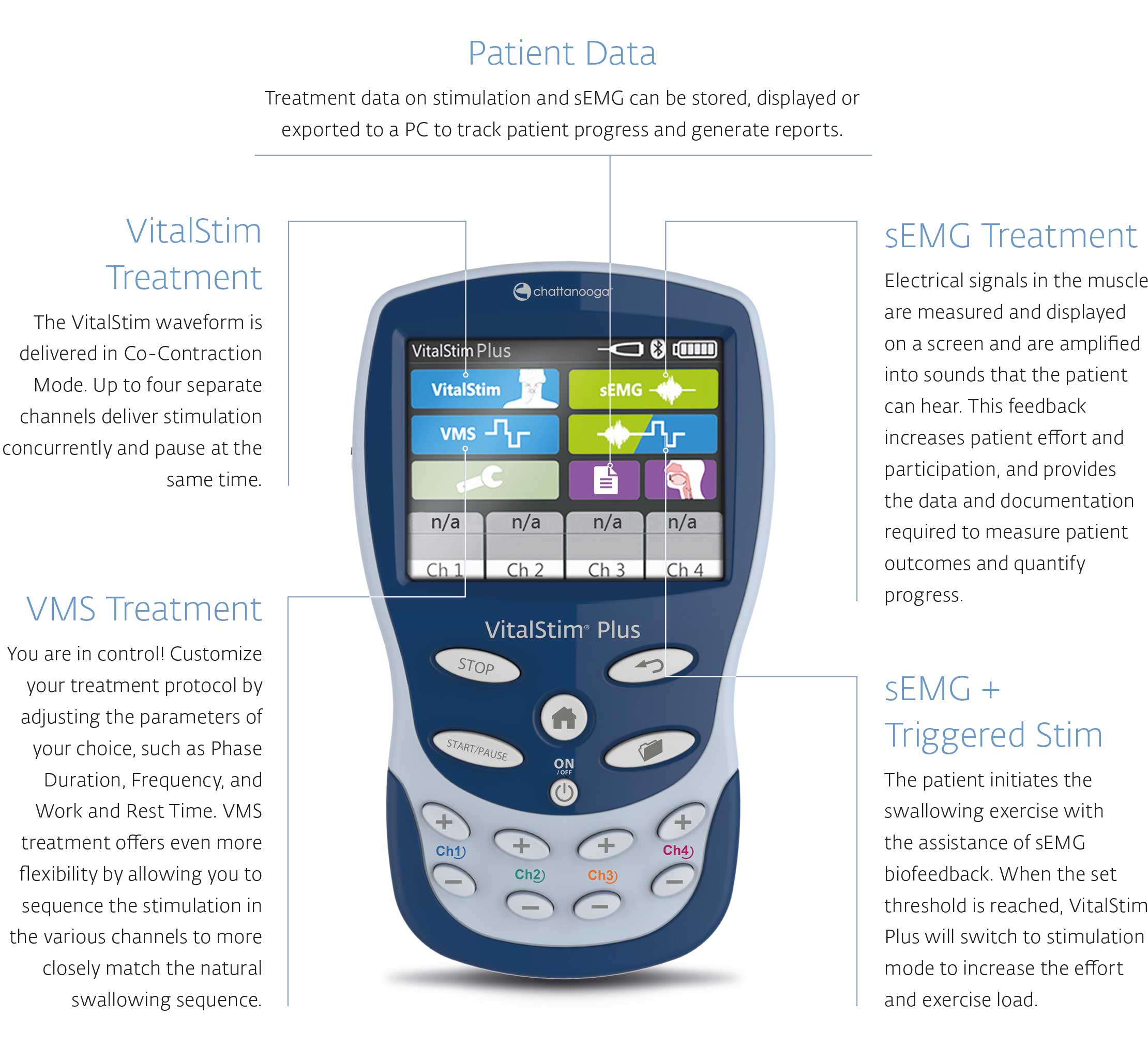
VitalStim Plus
Electrotherapy and sEMG Biofeedback system
Bluetooth Technology
Screen mirroring technology projects your live treatment onto a computer or tablet screen. This allows you to guide therapeutic exercise activities and increase patient engagement.
Education Videos
A complete education tool at yourfinger tips. VitalStim Plus includesvideos that help demonstrate keytherapeutic exercises, such as theMasako Exercise, the MendelsohnManeuver, the SupraglotticManeuver, and various ElectrodePlacements Options.
Anatomical Library
VitalStim Plus includes aDysphagia image library ofAnatomy, Pathology andTherapeutic Treatment options toassist in educating your patient.
